About Multiple Myeloma and M-Protein Monitoring
What is Multiple Myeloma?
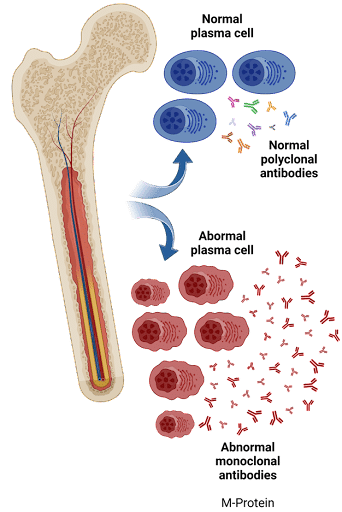
Multiple Myeloma (MM) is a cancer of the immune system caused by the uncontrolled proliferation of abnormal plasma cells in the bone marrow. In most cases of MM, large amounts of a single type of non-functional antibody protein (monoclonal immunoglobulin) are produced due to the expansion of a single, malignant plasma cell. These monoclonal immunoglobulins are called M-spike, paraprotein or M-protein.
FAQs.
M-protein is the unique product of malignant plasma cells. Combined with other signs and symptoms, the detection of M-protein can be used as a reliable marker to diagnose and monitor multiple myeloma disease burden.
Minimal residual disease is a term used to describe any cancer left in the body after treatment. Physicians use MRD as a measure of depth of response to a treatment and it has become an important parameter in assessing the disease burden in MM. MRD status has been correlated to progression-free survival and overall survival in the clinic.
Both SPEP and IFE are non-invasive, inexpensive and widely available blood tests for the routine detection and quantification of M-protein. However, their low sensitivity does not make them appropriate for MRD monitoring.
To measure MRD, highly sensitive tests, such as next generation flow cytometry (NGF) and next generation sequencing (NGS), are used to estimate the myeloma tumor burden by detecting any cancer cells from a standard sample of 1,000,000 cells (106 cells) taken from the bone marrow. However, these methods require painful bone marrow aspirations and occasionally result in false negative readings as a result of sampling bias due to the patchy nature of MM in the bone marrow.
Newly developed mass spectrometry-based blood tests, such as EasyM™, are able to combine the best advantages of the above mentioned approaches by being able to provide a simple non-invasive blood-based test that is as sensitive as bone marrow-based assays in order to reliably monitor MRD throughout treatment.
No, EasyM only requires a small drop of blood – as little as 0.1 mL.

