Convenient MRD Monitoring in the Blood for Myeloma Patients.
EasyM™ is an ultrasensitive multiple myeloma blood test that makes monitoring minimal residual disease (MRD) simpler, safer and more convenient.
A Novel Blood Test for MRD Monitoring in MM.
EasyM is a groundbreaking blood test for multiple myeloma MRD monitoring. EasyM uses the ultrasensitive clonotypic peptides mass spectrometry (MS) method to measure the amount of abnormal monoclonal antibody proteins (M-spike) made by your myeloma cells in the blood.
What is MRD and what does it mean?
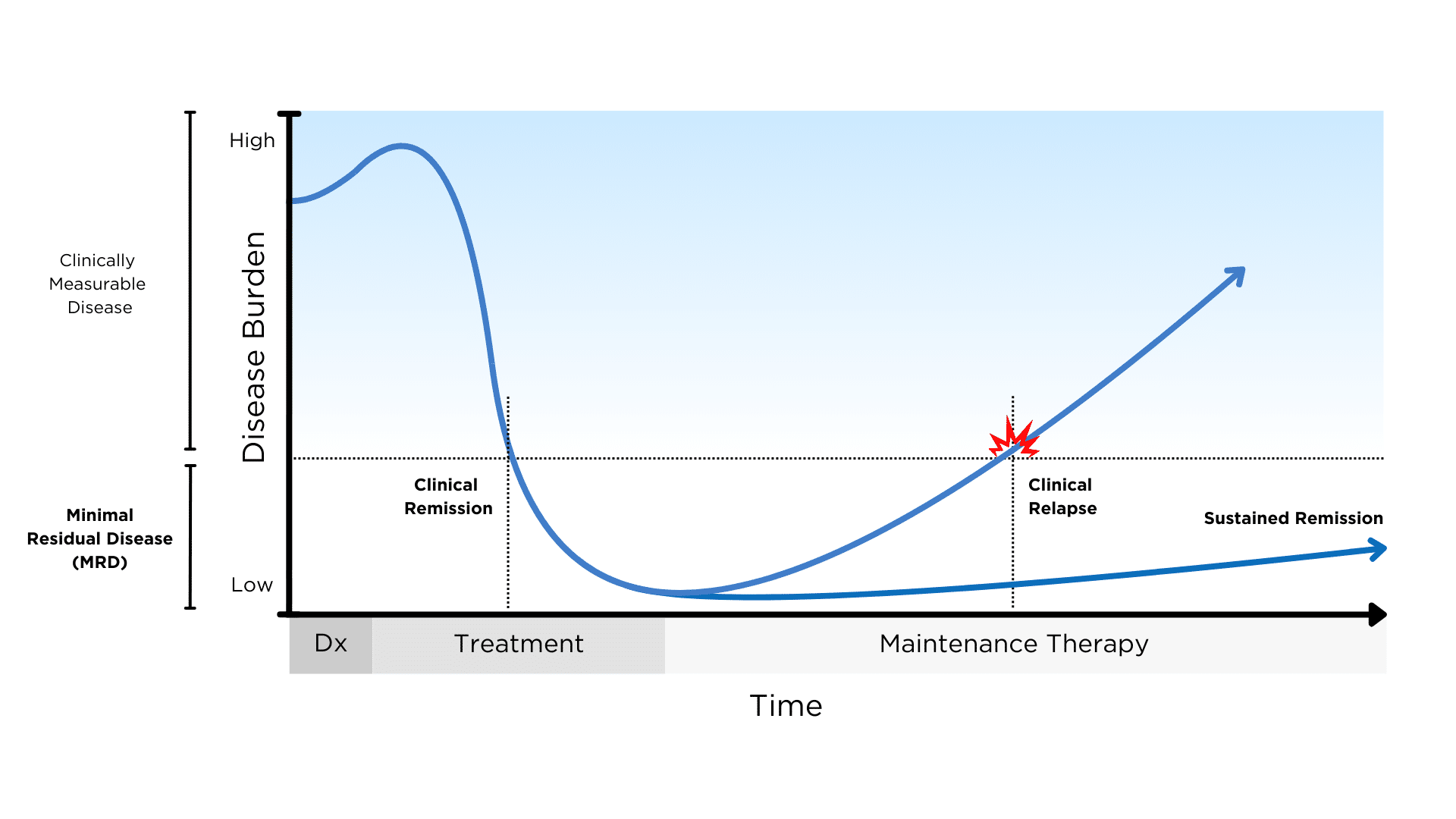
Minimal residual disease (MRD) describes the small number of cancer cells that remain in the body during or after treatment. The goal of MRD testing in multiple myeloma is to detect signs of residual disease in patients that might have been missed by less sensitive monitoring tests.
MRD negativity means that no myeloma cells are detected in a sample of 100,000 normal cells. MRD negativity has been linked to improved progression-free survival and overall survival in multiple myeloma, with deeper responses resulting in longer remissions. However, even MRD negative patients can still experience relapse, suggesting the need for even more sensitive tests.
Conventional MRD testing in myeloma is primarily done on samples obtained through invasive and painful bone marrow biopsy. Novel tests such as EasyM can conveniently and reliably monitor MRD in the blood without the need for bone marrow samples.
A Novel Blood Test for MRD Monitoring in MM.
EasyM is a groundbreaking blood test for multiple myeloma MRD monitoring. EasyM uses the ultrasensitive clonotypic peptides mass spectrometry (MS) method to measure the amount of abnormal monoclonal antibody proteins (M-spike) made by your myeloma cells in the blood.
What is MRD and what does it mean?
Minimal residual disease (MRD) describes the small number of cancer cells that remain in the body during or after treatment. The goal of MRD testing in multiple myeloma is to detect signs of residual disease in patients that might have been missed by less sensitive monitoring tests.
MRD negativity means that no myeloma cells are detected in a sample of 100,000 normal cells. MRD negativity has been linked to improved progression-free survival and overall survival in multiple myeloma, with deeper responses resulting in longer remissions. However, even MRD negative patients can still experience relapse, suggesting the need for even more sensitive tests.
Conventional MRD testing in myeloma is primarily done on samples obtained through invasive and painful bone marrow biopsy. Novel tests such as EasyM can conveniently and reliably monitor MRD in the blood without the need for bone marrow samples.
How does EasyM work?
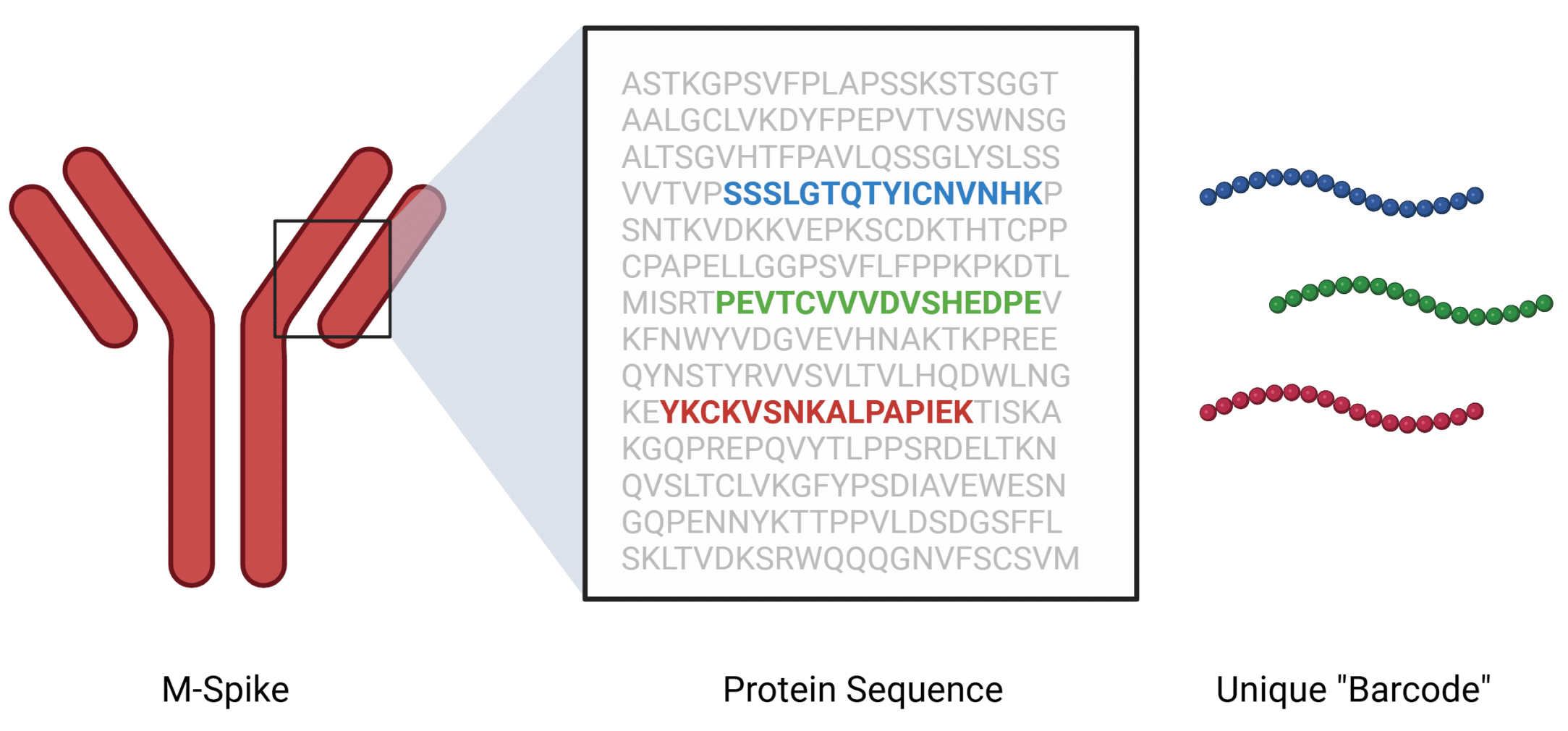
First, a baseline sample will be collected from you, usually early in your treatment when the M-spike is still abundant. This first sample will be sent to our lab for sequencing. EasyM uses mass spectrometry to obtain peptide sequences that are unique to your M-spike protein. These sequences act like a barcode that is used in follow up EasyM tests to track M-spike concentrations to levels 1000 times lower than standard tests such as serum protein electrophoresis (SPEP).
Why is the baseline sample important?
The unique M-spike peptide sequences are more reliably identified by mass spectrometry when the M-spike is abundant. This is why it is crucial to have the baseline sample sequenced as early as possible to give you and your care team the freedom to use EasyM at any time in the future.
Getting Started.
For patients interested in EasyM, please have your doctor contact us for further information.
We will work with your healthcare team to provide access to EasyM testing in your area.
EasyM is currently certified for clinical use under CLIA in all US states with the exception of NY. EasyM has not been cleared by the FDA and is not yet covered by commercial health insurance or Medicare/Medicaid.
Sign up for the latest updates.
Follow us on LinkedIn and/or sign up below to receive the latest EasyM news and its availability in your area as well as updates on our latest clinical trials..