Introduction
Multiple myeloma is a type of blood cancer that forms in plasma cells. Plasma cells, also known as plasmacytes, are a type of white blood cell located in the bone marrow that produce antibodies to fight infections.
While the general diagnosis is known as “multiple myeloma”, it actually comes in various forms, each with unique features that can influence prognosis and treatment. Here we will discuss the major types of multiple myeloma and what differentiates them.
What is Multiple Myeloma?
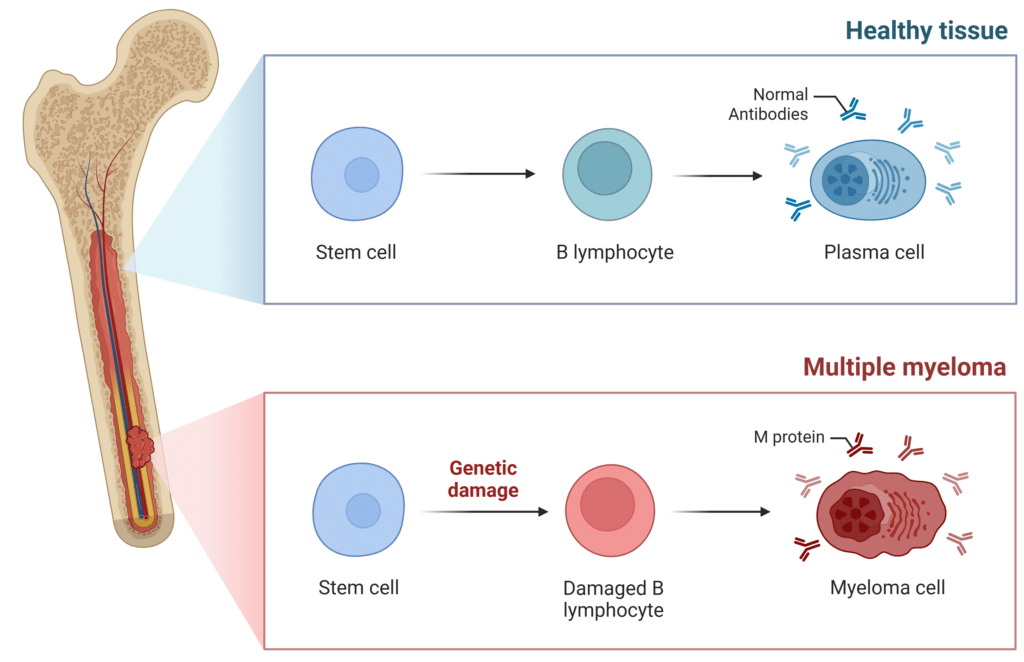
Multiple myeloma begins when abnormal plasma cells (myeloma cells) multiply uncontrollably in the bone marrow, crowding out healthy blood cells. Instead of making healthy antibodies to fight infections, myeloma cells often produce an abnormal monoclonal antibody, called M-protein (Figure 1).
Figure 1: Comparison of a Healthy Plasma Cell to a Cancerous Myeloma Cell.
M-Protein in Multiple Myeloma
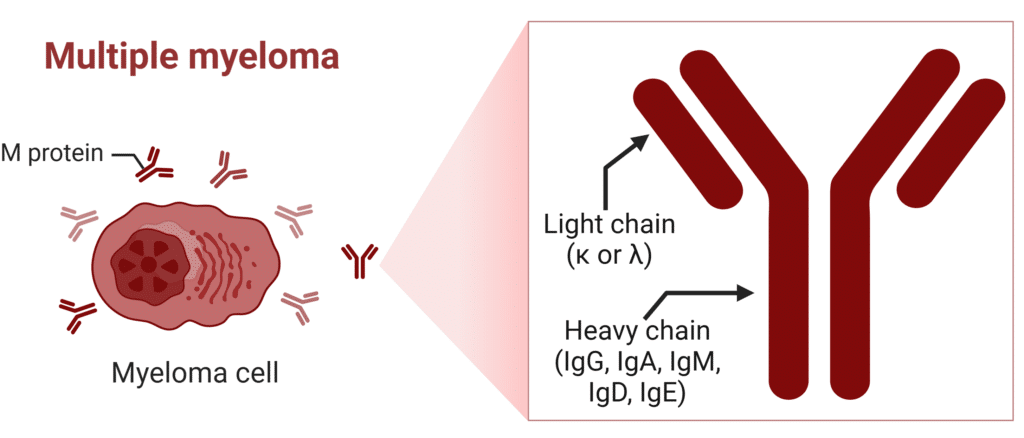
Multiple myeloma patients express a unique M-protein composed of a combination of heavy and light chains which can be detected in blood or urine and is used to diagnose and monitor the disease (Figure 2). The subtype of myeloma a patient has is typically classified based on the heavy chain produced by malignant plasma cells. The most common are IgG (60-65%) and IgA (20-25%) myelomas, while IgD (<2%) and IgE (<0.1%) are rare but clinically significant variants.
Figure 2: Structure of M-Protein in Multiple Myeloma (κ=kappa λ=lambda).
In total there are 10 possible myeloma subtypes:
- IgG lambda
- IgA lambda
- IgM lambda
- IgD lambda
- IgE lambda
- IgG kappa
- IgA kappa
- IgM kappa
- IgD kappa
- IgE kappa
Not every patient has the same subtype of multiple myeloma. Subtypes have been linked to differences in risk of progression and clinical features. Multiple myeloma can be further classified by the categories discussed below.
Clinical Variants of Multiple Myeloma
Smoldering Multiple Myeloma
Smoldering multiple myeloma (SMM) is an early, asymptomatic form of multiple myeloma that accounts for about 15% of all MM cases. This type of myeloma falls between the precancerous condition called monoclonal gammopathy of undetermined significance (MGUS) and active multiple myeloma.
SMM is diagnosed when patients have higher than normal levels of M-protein (≥30 g/L in the blood and/or ≥500 mg/24 hours in the urine) and/or abnormal plasma cells (10-60%), but without symptoms or evidence of organ damage (CRAB criteria or other myeloma defining events).
The risk of progression to active myeloma is evaluated based on several prognostic variables, including M-protein levels, M-protein type, presence of immunoparesis, serum free light chain (FLC) ratio, and underlying bone marrow or genetic abnormalities.
While observation remains the standard of care, patients identified as high risk for progression may be considered for early therapeutic intervention based on clinical judgment. The risk of a SMM patient progressing to multiple myeloma is approximately 10% per year for the first 5 years, 3% per year for the next 5 years, and 1-2% per year for the next 10 years.
Active Multiple Myeloma
Active multiple myeloma (MM) is the classic, symptomatic form of the disease. It typically evolves from precursor conditions such as MGUS or SMM. When MM is suspected clinically, the patient is tested for the presence of elevated M-protein. The current standard for M-protein testing is serum protein electrophoresis (SPEP) and serum immunofixation (SIFE).
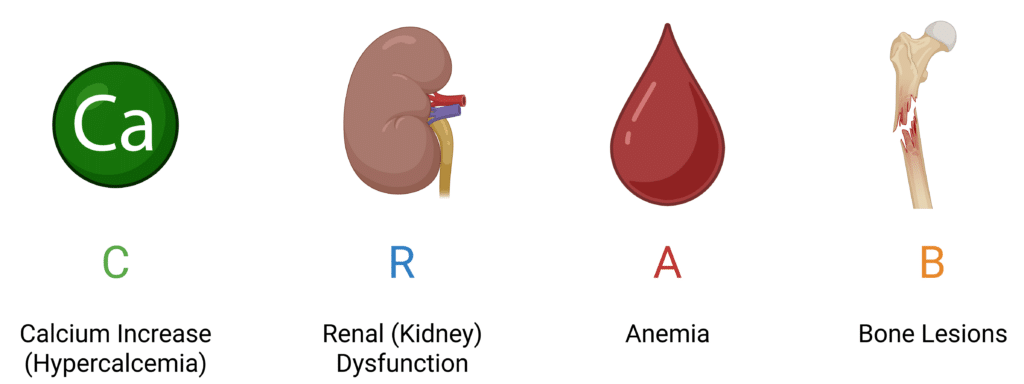
If M-protein is elevated, diagnosis is confirmed if clonal bone marrow plasma cells are ≥10% (or biopsy-proven bony/extramedullary plasmacytoma) as well as presentation of one or more of the “CRAB” criteria (Figure 3).
If a patient doesn’t display CRAB criteria, other myeloma defining events (MDEs) can provide a more in-depth diagnostic criteria. MDEs are ≥60% clonal plasma cells in the marrow, a serum free light chain ratio ≥100, or more than one focal lesion on MRI that is at least 5 mm in size.
Initial management of MM involves stabilization of any acute issues. Once stabilized, the treatment plan is dictated by the risk stratification and transplant eligibility. The presence of certain genetic abnormalities, such as p53 mutation, classifies a case as high risk multiple myeloma. Patients typically receive cycles of bortezomib, lenalidomide, and dexamethasone (VRd), with high risk patients potentially receiving daratumumab as part of Dara-VRd.
Figure 3: Hallmark Symptoms of Multiple Myeloma.
Non-Secretory Multiple Myeloma
Non-secretory multiple myeloma (NSMM) is a rare type of MM, accounting for approximately 1-2% of cases. Unlike typical MM, patients with NSMM do not produce detectable levels of M-protein.
Despite the absence of measurable M-protein, these patients exhibit the same malignant plasma cell proliferation in the bone marrow and are subject to the same MDEs, including CRAB criteria.
There are two main biological subtypes of NSMM:
- True non-producers with plasma cells that lack the ability to synthesize immunoglobulins.
- Non-secretors whose plasma cells produce immunoglobulins, but do not secret them into circulation or are rapidly degraded.
Diagnosis and monitoring often require a greater reliance on bone marrow biopsy and advanced imaging (e.g., PET-CT, MRI) which pose problems such as pain, invasiveness, and repeated exposure to radiation. Treatment for NSMM is typically the same as active myeloma and is guided by the same prognostic factors and clinical staging systems.
Light Chain Multiple Myeloma (Bence Jones)
In about 15% of multiple myeloma patients, the malignant myeloma cell does not produce a complete immunoglobulin. Light chain multiple myeloma (LCMM) is characterized by the production of either kappa (κ) or lambda (λ) light chains only, without the heavy chains (Figure 2).
In LCMM patients, the free light chains circulating in the blood are rapidly filtered by the kidneys. This can lead to accumulation in the kidneys causing damage. These light chains may also deposit in tissues and cause light chain deposition disease or lead to amyloidosis, causing further complications.
Diagnosis relies heavily on the serum free light chain (FLC) assay, which detects elevated levels and abnormal κ/λ ratios not picked up by standard serum or urine protein electrophoresis.
Clinically, patients with LCMM often present with features of organ damage, particularly renal insufficiency, and may have fewer lytic bone lesions than those with typical secretory myeloma. However, bone disease, anemia, and hypercalcemia may still occur. Treatment strategies align with those for active myeloma and typically include proteasome inhibitors, which are particularly effective at reducing free light chain levels and mitigating renal damage.
Oligosecretory Multiple Myeloma
Oligosecretory multiple myeloma (OSMM) is a less common subtype of myeloma in which malignant plasma cells produce low levels of monoclonal protein, insufficient for reliable detection or monitoring using standard serum or urine electrophoresis. This subtype represents an intermediate clinical entity between secretory and non-secretory myeloma.
The IMWG defines oligosecretory myeloma by a serum M-protein of ≥1 g/dL, or urine M-protein >200 mg/24 hours, and in some cases, ≥100 mg/L serum FLC. Although the M-protein level is low, OSMM patients typically experience MDEs, however, they have been found to have less renal dysfunction and bone marrow infiltration.
Much like non-secretory myeloma, diagnosis often requires greater reliance on bone marrow biopsy, serum FLC assay, and advanced imaging (MRI or PET-CT) to confirm and assess disease. Mass spectrometry methods are emerging as a non-invasive and highly sensitive way to detect and monitor M-protein levels that standard methods cannot detect. Treatment for OSMM is similar to active myeloma and is guided by the same prognostic factors and clinical staging systems.
Conclusion
Multiple myeloma requires personalized approaches to diagnosis, monitoring, and treatment. A key part of this process involves tracking M-proteins, which serve as important biomarkers for disease activity. Traditional tools like serum protein electrophoresis (SPEP) have helped physicians monitor these proteins, but their limited sensitivity is problematic.
Today, advanced mass spectrometry-based tests, like EasyM, offer a more precise and non-invasive way to detect M-protein, down to 0.00001 g/dL. These innovations have the potential to reshape how clinicians track treatment response and disease progression, supporting more tailored and effective care plans.
Disclaimer
This article is intended solely for educational and informational purposes and has not been reviewed by a licensed medical professional. It is not a substitute for professional medical advice, diagnosis, or treatment recommendations. Always seek the guidance of a qualified healthcare provider with any questions you may have regarding a medical condition. Do not ignore or delay seeking medical advice based on content presented here.