Introduction
As detection technology and therapeutics have improved, minimal residual disease (MRD) has become a pivotal prognostic marker in multiple myeloma (MM), particularly for predicting progression free and overall survival. In 2024, the FDA consensus is that MRD negativity can be used as a clinical trial endpoint. This shift is driving innovation in multiple myeloma blood tests, such as the highly sensitive mass spectrometry-based EasyM assay, which can allow for monitoring without the need for invasive bone marrow tests.
Publication Summary
This recently published review article provides a critical evaluation of MRD negative based decision making in multiple myeloma treatment plans, addressing both its potential benefits and limitations. The authors present a detailed overview of current MRD detection methods, including bone marrow-based next generation sequencing (NGS), next generation flow (NGF) cytometry, as well as novel peripheral blood-based test for multiple myeloma like the EasyM mass spectrometry assay.
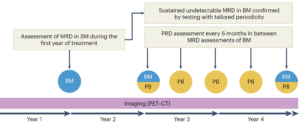
Overall, the authors believe that the path to curing multiple myeloma requires a deep and sustained MRD negative response. Their conclusion is that a combination of bone marrow and peripheral blood-based testing can be more informative than bone marrow alone. In fact, they suggest that peripheral blood (PB)-based testing can replace bone marrow aspirates in later stages of treatment (Figure 1).
Figure 1: Paiva et al. suggest a combination of bone marrow (BM) and peripheral blood (PB)-based MRD testing as a more informative and less invasive method.
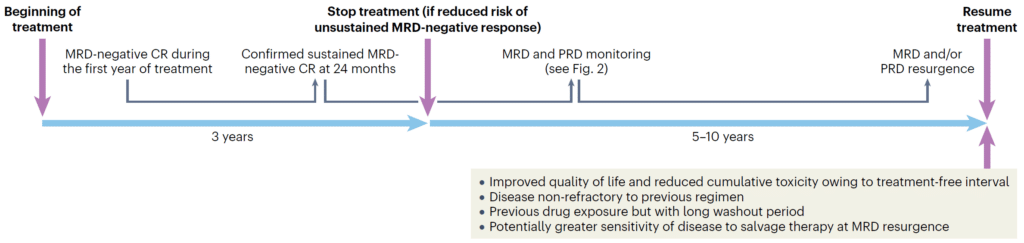
Of note, the authors highlight how frequent, highly sensitive peripheral blood based MRD testing, such as EasyM, could be used to guide drug treatment schedules (Figure 2). As seen in the GEM2014MAIN trial, patients were able to have a 5 year drug holiday due to undetectable MRD. This can significantly improve the patient’s quality of life, and as the authors state, bring us closer to a functional cure for multiple myeloma.
Figure 2: Paiva et al. present a hypothetical scenario of fixed treatment
duration in patients with sustained undetected MRD.
Key Takeaways
- Minimal residual disease (MRD) assessment is ready for prime time in the clinical management of multiple myeloma (MM).
- An MRD-negative complete response (CR) can be considered as the new definition of CR in MM.
- The efficacy of new regimens in inducing deeper and durable MRD-negative responses is clearly interconnected with prolonged survival.
- In 2024, consensus was reached on the role of MRD negativity as an early end point to support accelerated approval of new treatments for MM.
- The sequential use of MRD and peripheral residual disease (PRD) assays might be the next frontier of response assessment.
Conclusions
As multiple myeloma treatment continues to evolve, MRD assessment is emerging not just as a prognostic tool, but as a central component of clinical decision making. The integration of both bone marrow and peripheral blood-based testing, particularly through highly sensitive assays like EasyM, offers a more comprehensive and less invasive approach to monitoring disease. By enabling strategies such as prolonged drug holidays in patients with deep and sustained MRD negativity, this shift holds promise for improving quality of life and moving the field closer to achieving a functional cure.
Authors
Paiva, B., Shi, Q., Puig, N. et al. Opportunities and challenges for MRD assessment in the clinical management of multiple myeloma. Nat Rev Clin Oncol (2025). https://doi.org/10.1038/s41571-025-01017-x
Contact Us.
We want to make an impact on myeloma.
We welcome discussion with academic investigators, pharmaceutical companies, patient groups and related service providers. Please reach out.
Talk to Our Scientists.
We Have Sequenced 5000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics