Introduction
Minimal residual disease (MRD) is a critical prognostic indicator in multiple myeloma, typically assessed using invasive bone marrow-based assays. However, these methods have shown limited success when applied to blood. Next-generation flow (NGF) is mainly useful in patients with high levels of circulating disease, which is a phenomenon that has been associated with poor outcomes. Next-generation sequencing (NGS) tools, such as clonoSEQ, have poor limit-of-detection (LoD) in low disease states and do not work in extra-medullary disease contexts. As a result, there is a pressing need for a non-invasive, blood-based assay with sensitivity comparable to marrow-based techniques.
Clonotypic mass spectrometry (MS), which uses unique peptide fingerprints derived from a patient’s M-protein, offers a highly sensitive promising solution for blood-based MRD in Multiple Myeloma. This particular study evaluates EasyM clonotypic MS as a blood-based MRD tool in patients undergoing autologous hematopoietic cell transplant (AHCT) for multiple myeloma.
Publication Summary
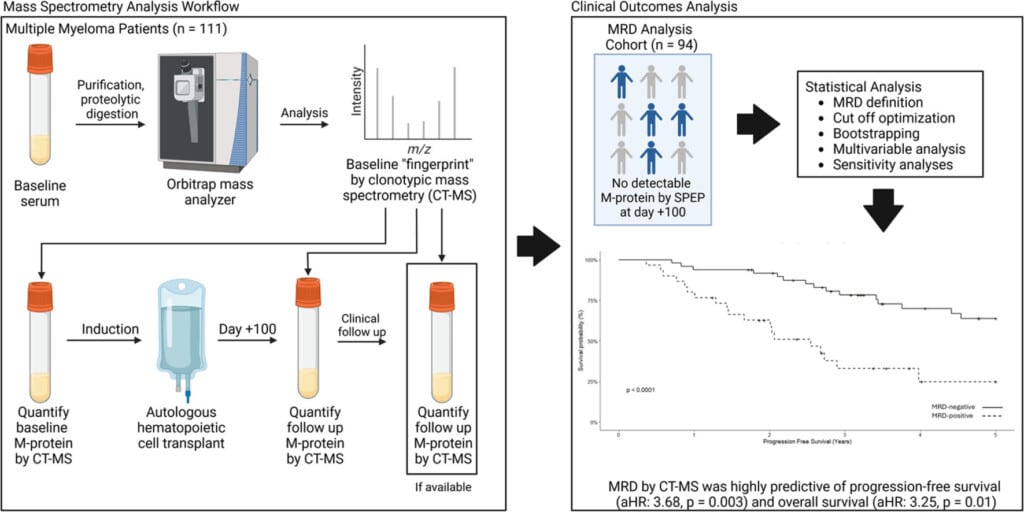
In this study, the performance and prognostic value of clonotypic MS MRD testing was assessed in post-AHCT multiple myeloma patients. By identifying and tracking patient specific signatures from serum samples, the authors demonstrate that EasyM clonotypic MS MRD measurement has strong predictive power for progression free survival (PFS) and overall survival (OS) (Figure 1).
Figure 1: Illustration of clonotypic MS MRD testing workflow and clinical outcomes analysis (graphical abstract from Slade et al., 2025).
Key Findings
- Clonotypic signatures were successfully generated from baseline serum in nearly all patients (99%), confirming high feasibility. The approach detects disease with a sensitivity significantly greater than conventional blood-based methods.
- EasyM Clonotypic MS blood-based MRD negativity, as early as Day 100 post AHCT, strongly predicted improved progression free survival (PFS) and overall survival (OS), performing comparably to marrow-based MRD assays.
- EasyM MRD status remained a significant prognostic factor independent of traditional MM markers.
- The clonotypic fingerprint was stable in 95% of patients and remained a reliable disease marker over years, suggesting long term utility.
Conclusions
This study demonstrates that EasyM clonotypic MS is a feasible and powerful blood-based MRD assay with prognostic accuracy on par with invasive bone marrow-based tests. Its ability to non-invasively monitor disease with higher frequency than bone marrow-based assays even years post-baseline, underscores its clinical utility. While more work is needed to refine optimal sampling time points, clonotypic MS holds great promise for less invasive, biopsy-free MRD assessment in multiple myeloma management.
Authors
Michael J. Slade MD, MSCI , Julie Fortier PhD , Abir Khaled PhD , Mark Fiala PhD , Zac McDonald PhD , Mark A. Zaydman MD, PhD , Fei Wan PhD , Mark A. Schroeder MD , Keith Stockerl-Goldstein MD , Liqiang Yang PhD , Ravi Vij MD, MBA , Blood-based Measurable Residual Disease by Clonotypic Mass Spectrometry is Prognostic in Patients with Multiple Myeloma Undergoing Autologous Hematopoietic Cell Transplant, Clinical Lymphoma, Myeloma and Leukemia (2025), doi: https://doi.org/10.1016/j.clml.2025.04.017
Contact Us.
We want to make an impact on myeloma.
We welcome discussion with academic investigators, pharmaceutical companies, patient groups and related service providers. Please reach out.
Talk to Our Scientists.
We Have Sequenced 5000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics