Introduction
Once initial treatment of multiple myeloma is complete, maintenance therapy, often involving lenalidomide or proteasome inhibitors, is the standard of care. However, maintenance therapy can cause a number of negative side effects, such as toxicity, thereby reducing quality of life.
Minimal residual disease (MRD) is a key predictor of outcomes in multiple myeloma. Greater assay sensitivity has been shown to improve prognostic accuracy. While a single MRD negative result is useful, sustained MRD negativity offers even stronger evidence of durable remission and better survival prospects.
Considering the above, researchers are asking the question of whether or not patients with sustained MRD negativity can safely stop maintenance therapy, challenging the need for indefinite treatment for multiple myeloma patients.
Publication Summary
The MRD2STOP phase II clinical trial focused on determining whether patients with multiple myeloma who achieve sustained multimodal MRD negativity can safely discontinue maintenance therapy without negatively affecting outcomes.
To do this, adult patients with multiple myeloma were enrolled if they received maintenance therapy, reached complete response (CR) by IMWG criteria, no detectable disease by PET scan, and demonstrated a sustained MRD negativity. Patients with durable MRD negativity could elect to stop maintenance therapy and then were followed to monitor relapse or disease progression.
Although the goal was to test the feasibility and safety of stopping maintenance therapy in MRD negative patients there were secondary goals that included monitoring progression free survival (PSF), overall survival (OS), quality of life (QoL), toxicity reduction and biomarkers correlations.
Key Takeaways
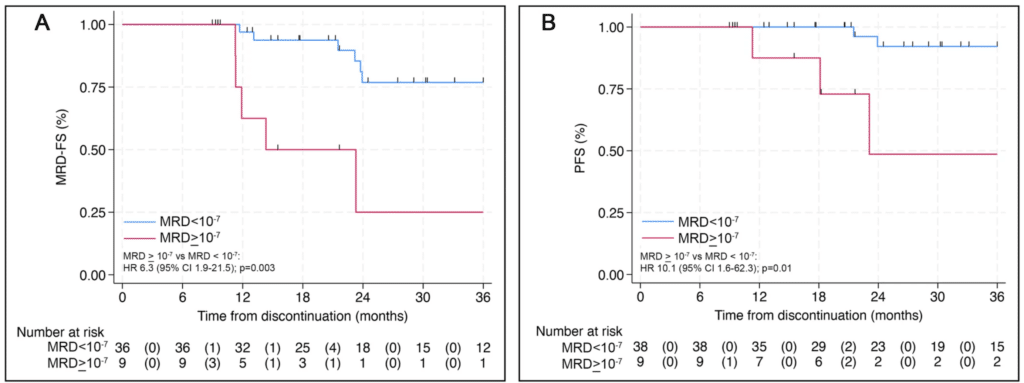
- In patients with multiple myeloma, discontinuation of maintenance therapy after achieving deep, sustained MRD negativity (<10⁻⁶) resulted in a 3-year progression-free survival (PFS) of 92%, with no immediate safety concerns (Figure 1).
- Deeper MRD negativity correlated with improved outcomes and quality of life, suggesting that MRD-guided therapy discontinuation is both feasible and beneficial.
- Though long-term survival data are still evolving, early evidence indicates that this approach does not lead to rapid relapse and may reduce treatment-related toxicity while preserving remission.
- Incorporating highly sensitive MRD assays (e.g., <10⁻⁷), advanced imaging, and peripheral blood tests, such as clonotypic mass spectrometry, could enhance detection of deep responses and inform more personalized, potentially curative treatment strategies.
Figure 1: 10⁻⁷ MRD Status Outcomes. A MRD-free survival (MRD-FS) and B progression-free survival (PFS) stratified by MRD 10−7 status at baseline. MRD-FS refers to patients free of MRD 10−6 resurgence, progression, or death.
Conclusions
MRD2STOP clinical trial provides promising evidence that using MRD as a treatment endpoint can personalize therapy more precisely. This aligns with the broader trend in hematologic oncology of shifting from treating indefinitely to treat until deep remission is achieved.
EasyM and Discontinuation of MM Maintenance Therapy
The authors of this publication suggested that using complementary measurements of MRD, including clonotypic peptide mass spectrometry, may further enhance understanding. EasyM is a blood based MRD assay using the clonotypic peptide mass spectrometry approach. EasyM was not included in the MRD2STOP trial, however other clinical trials are now exploring the utility of EasyM in this context. If you would like to know more about studies of MRD guided discontinuation of maintenance therapy, please contact us.
Authors
Derman, B.A., Major, A., Cooperrider, J. et al. Discontinuation of maintenance therapy in multiple myeloma guided by multimodal measurable residual disease negativity (MRD2STOP). Blood Cancer J. 14, 170 (2024). https://doi.org/10.1038/s41408-024-01156-x
Contact Us.
We want to make an impact on myeloma.
We welcome discussion with academic investigators, pharmaceutical companies, patient groups and related service providers. Please reach out.
Talk to Our Scientists.
We Have Sequenced 5000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics